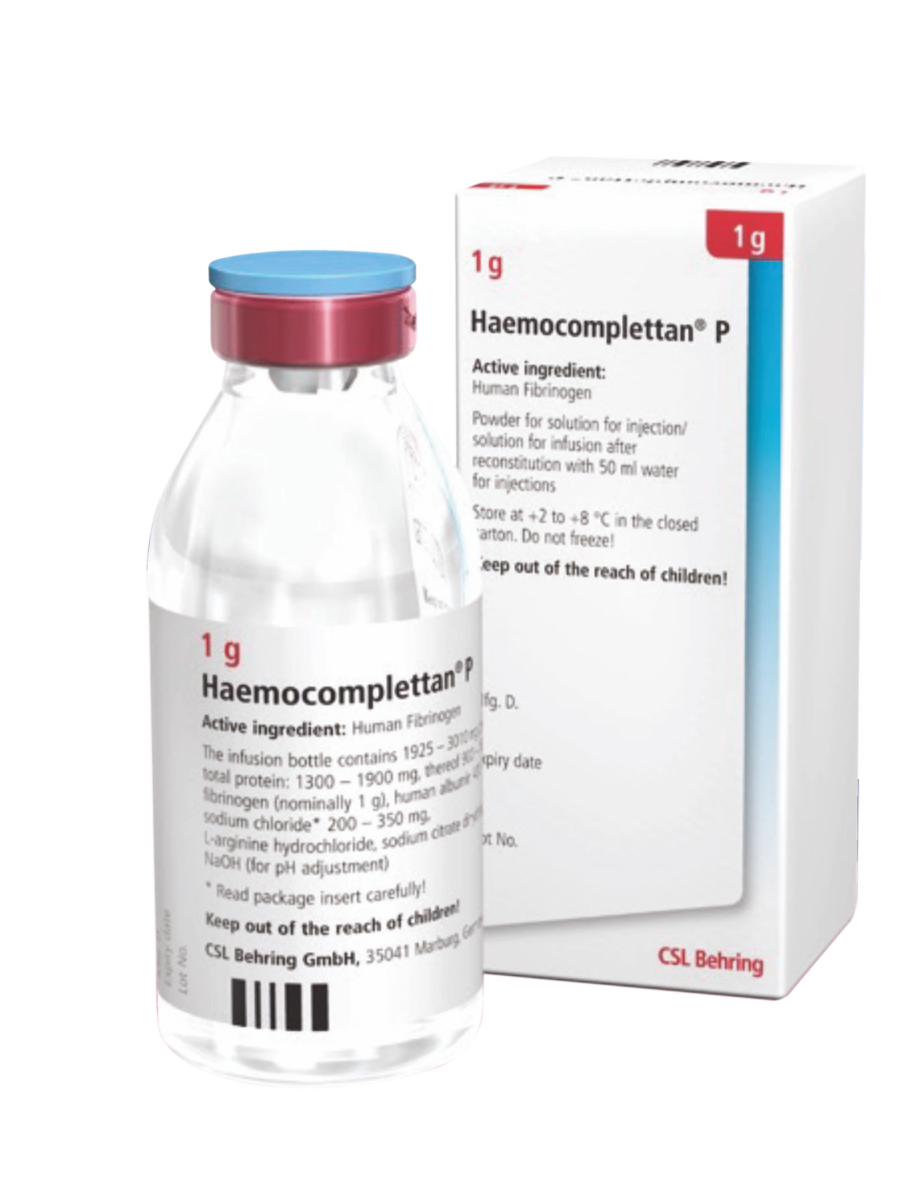
Product Description
Haemocomplettan P :Presented as a powder for solution for injection or infusion for intravenous administration
Indications
Therapy and prophylaxis of haemorrhagic diatheses in
- Congenital hypo-, dys- or afibrinogenaemia
- Acquired hypofibrinogenaemia resulting from
- disorders of synthesis in cases of severe liver parenchyma damage
- increased intravascular consumption e.g. as a result of disseminated intravascular coagulation, hyperfibrinolysis
- increased blood loss
Storage
- Store at 2 to 8°C
- Protect from light
- Do not freeze.
Shelf-life
60 months from the date of manufacture
Virus safety
Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses.
No cases of viral transmission reported in post-marketing safety data of > 27 years
1. India approved prescribing information for Haemocomplettan ® P, February 2021
2. Solomon C, et al. Thromb Haemost. 2015;113(4):759-771.
Medical code:IND-HCT-0025